
Mark Nicolls, MD
From Bench to Bedside for Pulmonary Hypertension

Mark Nicolls, MD
From Bench to Bedside for Pulmonary Hypertension
For 15 years, Mark Nicolls, MD — a pulmonary and critical care doctor and researcher — has been studying pulmonary arterial hypertension (PAH), a rare form of high blood pressure in the lungs. The affected arteries stiffen and thicken, making it hard for the heart to pump blood to the lungs. Today, there’s no cure for the disease, and patients have a limited life expectancy. But Nicolls hopes to change that, and his basic research has led to a drug now being investigated by a publicly-traded pharmaceutical company.
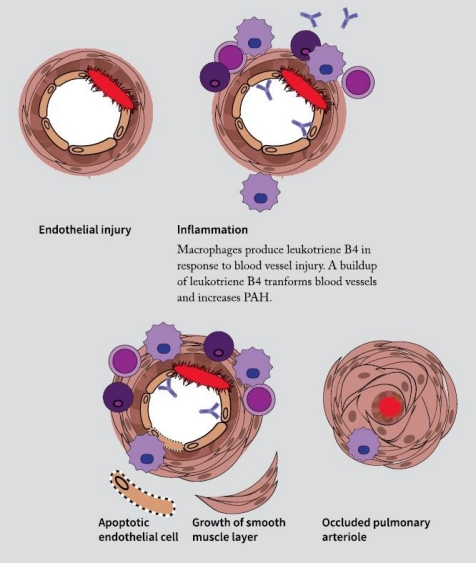
By studying the blood vessels that are injured in PAH at a molecular level, Nicolls and his lab group discovered that immune cells called macrophages tended to cluster in the vessels. Coincidentally, just as they made this finding, a new member of the lab, Amy Tian, PhD, was looking for a project. Her background was in eicosanoids, a type of signaling molecule used by the immune system. That background proved valuable when she began to study the signaling involved in the immune cells congregating in PAH-affected vessels.
“By looking at macrophages around the injured blood vessels, she was pretty quickly able to discern that they were synthesizing a lot of leukotriene B4,” says Nicolls. Leukotriene B4 is an eicosanoid, known to be produced in response to inflammation.
Tian and Nicolls showed that leukotriene B4 wasn’t just a consequence of PAH; it was part of the cycle of inflammation and injury that keeps the disease progressing. When they blocked leukotriene B4 in rats with the disease, their symptoms lessened and blood vessels became less clogged, lowering blood pressure in the lungs. Their results were published in the August 28, 2013, issue of Science Translational Medicine.
Shortly afterward, the researchers turned to Stanford’s SPARK program, a partnership between academia and industry that helps advance research discoveries to clinical trials and commercialization.
“We’re a translational research program, and we work with faculty, post-docs and students who have discoveries that might be turned into drugs for unmet medical needs,” explains Kevin Grimes, co-director of SPARK. “There are a lot of discoveries that never leave universities because they’re considered too risky by potential commercial partners. The expense and time and know-how of getting to the point where a commercial partner would be interested is just perceived to be huge.” The program provides funding, mentorship and education to bridge that gap from bench to bedside.
Blocking leukotriene B4 to treat PAH fit the bill, and Tian and Nicolls started working with Grimes. “Their work is really nice and innovative,” says Grimes. One of the selling points that helped move it along: A drug already existed that blocked leukotriene B4 and had been used on patients in Japan for a different condition.
For 15 years, Mark Nicolls, MD — a pulmonary and critical care doctor and researcher — has been studying pulmonary arterial hypertension (PAH), a rare form of high blood pressure in the lungs. The affected arteries stiffen and thicken, making it hard for the heart to pump blood to the lungs. Today, there’s no cure for the disease, and patients have a limited life expectancy. But Nicolls hopes to change that, and his basic research has led to a drug now being investigated by a publicly-traded pharmaceutical company.
By studying the blood vessels that are injured in PAH at a molecular level, Nicolls and his lab group discovered that immune cells called macrophages tended to cluster in the vessels. Coincidentally, just as they made this finding, a new member of the lab, Amy Tian, PhD, was looking for a project. Her background was in eicosanoids, a type of signaling molecule used by the immune system. That background proved valuable when she began to study the signaling involved in the immune cells congregating in PAH-affected vessels.
“By looking at macrophages around the injured blood vessels, she was pretty quickly able to discern that they were synthesizing a lot of leukotriene B4,” says Nicolls. Leukotriene B4 is an eicosanoid, known to be produced in response to inflammation.
Tian and Nicolls showed that leukotriene B4 wasn’t just a consequence of PAH; it was part of the cycle of inflammation and injury that keeps the disease progressing. When they blocked leukotriene B4 in rats with the disease, their symptoms lessened and blood vessels became less clogged, lowering blood pressure in the lungs. Their results were published in the August 28, 2013, issue of Science Translational Medicine.
Shortly afterward, the researchers turned to Stanford’s SPARK program, a partnership between academia and industry that helps advance research discoveries to clinical trials and commercialization.
By collaborating with Stanford’s SPARK program as well as those outside the university, Stanford clinician-scientist Mark Nicolls has moved a drug into clinical trials.
“We’re a translational research program, and we work with faculty, post-docs and students who have discoveries that might be turned into drugs for unmet medical needs,” explains Kevin Grimes, co-director of SPARK. “There are a lot of discoveries that never leave universities because they’re considered too risky by potential commercial partners. The expense and time and know-how of getting to the point where a commercial partner would be interested is just perceived to be huge.” The program provides funding, mentorship and education to bridge that gap from bench to bedside.
Blocking leukotriene B4 to treat PAH fit the bill, and Tian and Nicolls started working with Grimes. “Their work is really nice and innovative,” says Grimes. One of the selling points that helped move it along: A drug already existed that blocked leukotriene B4 and had been used on patients in Japan for a different condition.
“They’re repurposing a drug that has already been used in humans,” says Grimes. “The fact that there was a safety track record has allowed movement into the clinic to go more rapidly.” With the help of SPARK, Tian and Nicolls were able to get commercial interest in their discovery.
In mid-2016, following FDA approval, Eiger BioPharmaceuticals, Inc. launched the first clinical trial of the drug to treat patients with PAH at 45 sites throughout the United States and Canada. Nicolls is a scientific advisor for the company. “The fact that Mark has moved into the clinic so quickly is really a fantastic achievement,” says Grimes.
It remains to be seen how the drug works in patients, but Nicolls has high hopes. “The main therapeutic approach right now is vasodilation, which really treats the symptoms and not the disease. We’re hopeful that this therapy might actually reverse the disease,” says Nicolls.
By collaborating with Stanford’s SPARK program as well as those outside the university, Stanford clinician-scientist Mark Nicolls has moved a drug into clinical trials.

“They’re repurposing a drug that has already been used in humans,” says Grimes. “The fact that there was a safety track record has allowed movement into the clinic to go more rapidly.” With the help of SPARK, Tian and Nicolls were able to get commercial interest in their discovery.
In mid-2016, following FDA approval, Eiger BioPharmaceuticals, Inc. launched the first clinical trial of the drug to treat patients with PAH at 45 sites throughout the United States and Canada. Nicolls is a scientific advisor for the company. “The fact that Mark has moved into the clinic so quickly is really a fantastic achievement,” says Grimes.
It remains to be seen how the drug works in patients, but Nicolls has high hopes. “The main therapeutic approach right now is vasodilation, which really treats the symptoms and not the disease. We’re hopeful that this therapy might actually reverse the disease,” says Nicolls.
