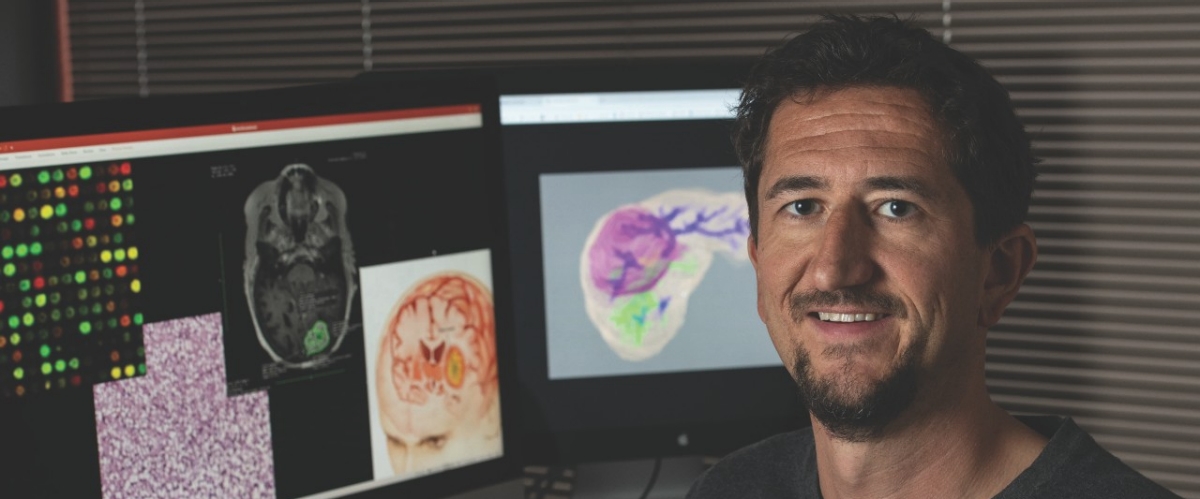
Now that computers can be taught to process large amounts of data and to recognize patterns in them, their usefulness in medicine is greatly enhanced.
In the hands of Olivier Gevaert, PhD, assistant professor of biomedical informatics, patients with a variety of diseases including cancers, neurodegenerative diseases, and cardiovascular diseases are being helped without even knowing it, thanks to artificial intelligence.
Gevaert fuses data from disparate sources to create algorithms to guide clinicians making diagnoses, prognoses, and treatment decisions. Since medical knowledge is said to double every few months, there will always be a plethora of data for him and his colleagues to work with.
About the methods he uses to study reams of data, Gevaert says, “I see them as different tools in the toolbox of machine learning. Some of them have more of a statistical flavor, some are more mathematical, some are pure machine learning. They are all part of the big brother field of machine learning.”
From Cancers in General to Specific Cancers
Besides using different tools, Gevaert and his colleagues use many different types of data: radiographic images, genetics, clinical data, even economic data. Much of this work has been focused on cancers since he came to Stanford as a postdoctoral fellow in 2010 after completing his master’s and PhD at the University of Leuven in Belgium.
“For example,” he says, “we developed computational algorithms for identifying cancer-causing genes using multi-omics data from genes, molecules, and proteins, among others. We use any type of machine-learning algorithm to integrate these different types of data.”
In addition to employing omics data, those in Gevaert’s lab fuse multiscale biomedical data—bridging the molecular-using omics data, the cellular-using pathology data, and tissue-using medical imaging data. They hope to learn which data source is most predictive of diagnosis, treatment, outcome, and prognosis.
Now that computers can be taught to process large amounts of data and to recognize patterns in them, their usefulness in medicine is greatly enhanced.
In the hands of Olivier Gevaert, PhD, assistant professor of biomedical informatics, patients with a variety of diseases including cancers, neurodegenerative diseases, and cardiovascular diseases are being helped without even knowing it, thanks to artificial intelligence.
Gevaert fuses data from disparate sources to create algorithms to guide clinicians making diagnoses, prognoses, and treatment decisions. Since medical knowledge is said to double every few months, there will always be a plethora of data for him and his colleagues to work with.
About the methods he uses to study reams of data, Gevaert says, “I see them as different tools in the toolbox of machine learning. Some of them have more of a statistical flavor, some are more mathematical, some are pure machine learning. They are all part of the big brother field of machine learning.”
From Cancers in General to Specific Cancers
Besides using different tools, Gevaert and his colleagues use many different types of data: radiographic images, genetics, clinical data, even economic data. Much of this work has been focused on cancers since he came to Stanford as a postdoctoral fellow in 2010 after completing his master’s and PhD at the University of Leuven in Belgium.
“For example,” he says, “we developed computational algorithms for identifying cancer-causing genes using multi-omics data from genes, molecules, and proteins, among others. We use any type of machine-learning algorithm to integrate these different types of data.”
In addition to employing omics data, those in Gevaert’s lab fuse multiscale biomedical data—bridging the molecular-using omics data, the cellular-using pathology data, and tissue-using medical imaging data. They hope to learn which data source is most predictive of diagnosis, treatment, outcome, and prognosis.
Importantly, says Gevaert, “You can imagine that if you treat each data source in isolation, you will have some predictive value. But what happens if we put them together? Is the sum greater than the parts?”
“We did one study where we showed that combining clinical data, genomic activity, imaging, and pathology data improved our ability to predict outcomes for a number of cancers.”
Transfer Learning to Pre-train a Model
If Gevaert and his colleagues are able to make their toolbox more generic and flexible, it can be used in different disease areas. Because the models that they train are very complex, they need a lot of data.
“What we’re trying to do,” he explains, “is called transfer learning, which means we’re using data in one disease area to train the models before we transfer them to another disease area where we have fewer data. This is pre-training.”
Using thousands of MRI images from a large cohort of healthy people and people with neurodegenerative diseases, for example, they can pre-train a model so it knows what a brain is and what an MRI image looks like. And then they can further train it using a cohort of as few as 200 brain tumor patients at Stanford.
For the past year, the Gevaert lab has also focused on cardiovascular diseases. For now they are most interested in diagnostics. “We have some preliminary results where we have looked at labs and symptoms in patients over time,” he explains. “We have clinical records of all Stanford patients for the past 15 years and we have looked at a subset of about 150,000 patients with up to 20 cardiovascular diseases. We’re now trying to distinguish them from people who are healthy.”
Artificial intelligence has opened many doors for study within the health care realm. And Olivier Gevaert and his colleagues will walk through as many of those doors as possible.
Importantly, says Gevaert, “You can imagine that if you treat each data source in isolation, you will have some predictive value. But what happens if we put them together? Is the sum greater than the parts?”
“We did one study where we showed that combining clinical data, genomic activity, imaging, and pathology data improved our ability to predict outcomes for a number of cancers.”
Transfer Learning to Pre-train a Model
If Gevaert and his colleagues are able to make their toolbox more generic and flexible, it can be used in different disease areas. Because the models that they train are very complex, they need a lot of data.
“What we’re trying to do,” he explains, “is called transfer learning, which means we’re using data in one disease area to train the models before we transfer them to another disease area where we have fewer data. This is pre-training.”
Using thousands of MRI images from a large cohort of healthy people and people with neurodegenerative diseases, for example, they can pre-train a model so it knows what a brain is and what an MRI image looks like. And then they can further train it using a cohort of as few as 200 brain tumor patients at Stanford.
For the past year, the Gevaert lab has also focused on cardiovascular diseases. For now they are most interested in diagnostics. “We have some preliminary results where we have looked at labs and symptoms in patients over time,” he explains. “We have clinical records of all Stanford patients for the past 15 years and we have looked at a subset of about 150,000 patients with up to 20 cardiovascular diseases. We’re now trying to distinguish them from people who are healthy.”
Artificial intelligence has opened many doors for study within the health care realm. And Olivier Gevaert and his colleagues will walk through as many of those doors as possible.
