Cancer’s Challenger: Allison Betof Warner and the Future of Cancer Treatment
#Methods
Mark and Mary Stevens Scholar Allison Betof Warner, MD, PhD, in her laboratory, pioneering advancements in melanoma treatment through Tumor-Infiltrating Lymphocyte (TIL) Therapy
Allison Betof Warner, MD, PhD, has never been one to shy away from challenges. When she was growing up outside of Philadelphia, her early years as a high-level gymnast instilled a determination to tackle difficult tasks head-on – a trait that has guided her through the world of medicine and research.
“I think being a gymnast gave me the drive to do really challenging things and take on the hard problems,” Betof Warner reflects, tracing the origins of her journey from athlete to oncologist.
Betof Warner, who was the first in her family to enter the medical field, decided at 10 years old that her future lay in medical research. This early resolution steered her through an impressive academic journey – from Cornell, balancing premed studies and gymnastics, to Duke, where she pursued an MD-PhD focused on cancer biology. It was during her residency at Massachusetts General Hospital that Betof Warner’s path took a crucial turn.
There, she first encountered immunotherapy, then an emerging treatment that uses the body’s immune system to fight cancer. This experience solidified her fascination with oncology – a field where innovations could rapidly transition from the lab to clinical use, offering new hope to patients.
Immunotherapy, particularly treatments known as checkpoint inhibitors, was a revelation to Betof Warner. Unlike traditional chemotherapy, which can be relentless and without a guarantee of lasting effectiveness, immunotherapy offers the possibility of long-lasting responses from limited durations of treatment.
How Do Checkpoint Inhibitors Work?
T cells’ inherent job in the body is to kill things that don’t belong. That can include viruses, bacteria, or even cancer. “Cancer is good at developing ways to hide,” explains Betof Warner. “I often compare this to Harry Potter’s invisibility cloak – cancer puts on an invisibility cloak to evade detection. The purpose of checkpoint inhibitor drugs is to remove that cloak, allowing the immune cells, specifically T cells, to see and attack the cancer. These T cells have the ability to kill cancer; what they lacked was the ability to see it.
“I fell in love with the idea that we could harness the immune system to create durable responses for patients,” she says. “It meant that patients wouldn’t necessarily need to be on treatment forever. We were controlling advanced cancer and getting patients back to their lives.”
“I fell in love with the idea that we could harness the immune system to create durable responses for patients,” she says. “It meant that patients wouldn’t necessarily need to be on treatment forever. We were controlling advanced cancer and getting patients back to their lives.”
– Allison Betof Warner, MD, PhD, Mark and Mary Stevens Scholar
Leading a New Frontier at Stanford: Solid Tumor Cell Therapy With TILs
Though checkpoint inhibitors have improved cancer care, the majority of patients still do not achieve long-term disease control with this approach. Cellular therapy is a different strategy that utilizes expanded and/or engineered T cells to incite a durable immune response to cancer.
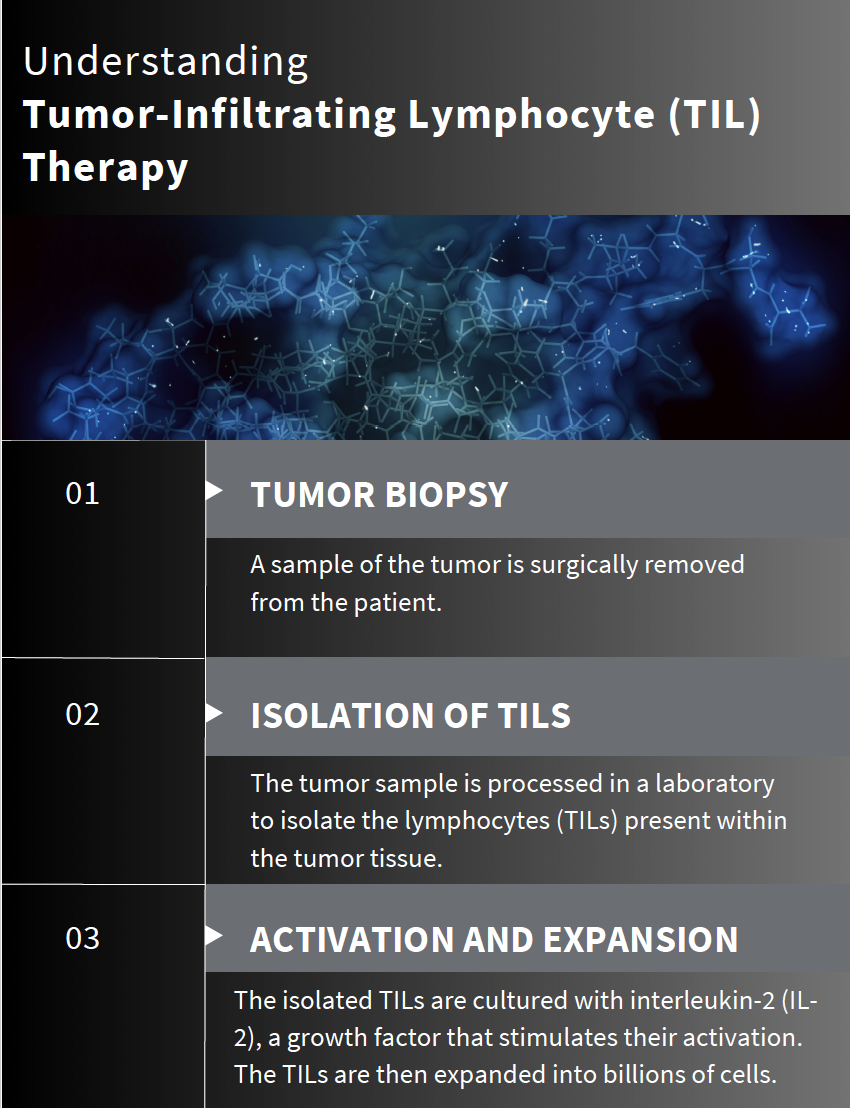
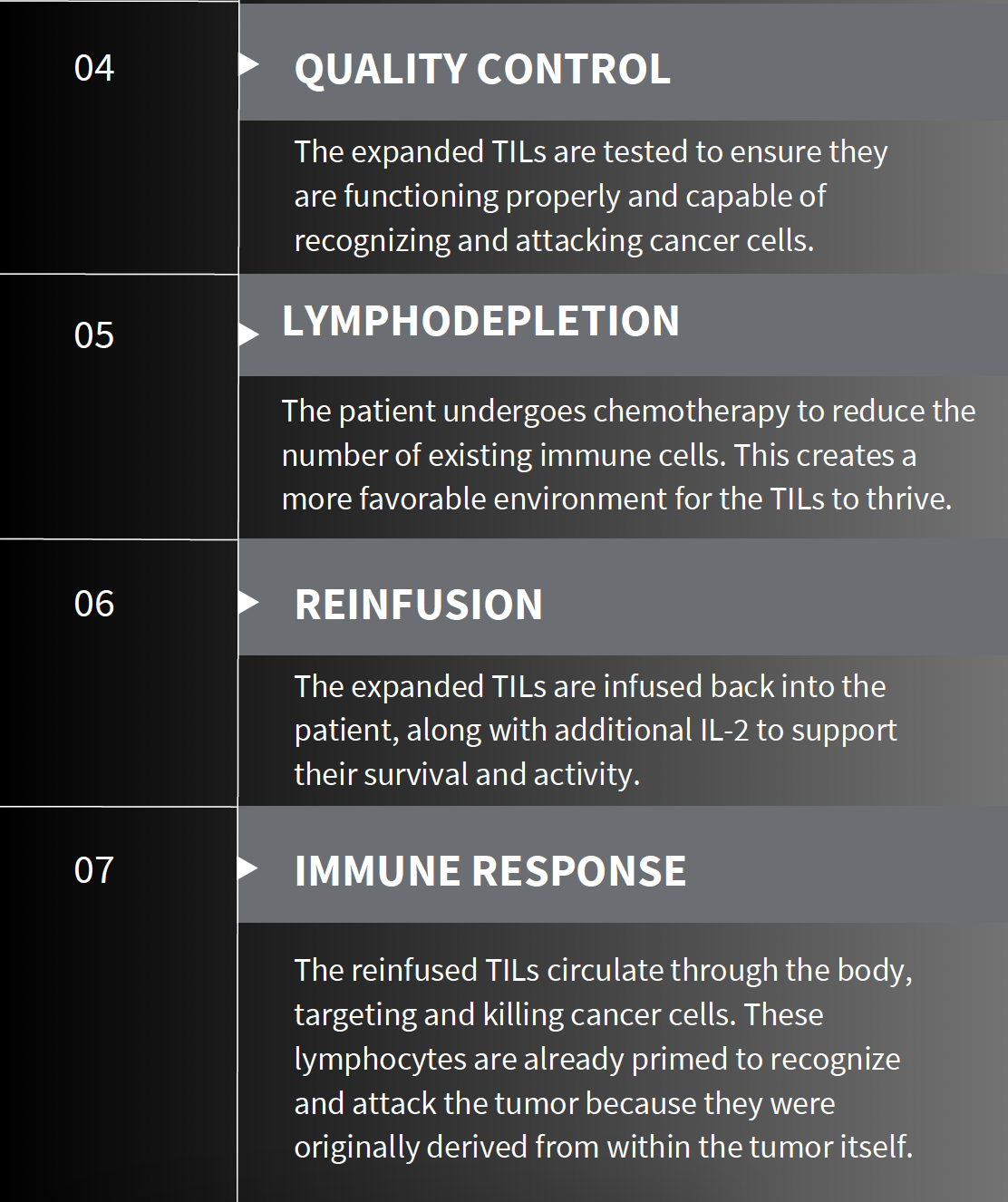
At Stanford, Betof Warner leads the advanced melanoma group and oversees the development of solid tumor cell therapies. She has been at the forefront of developing a new method for treating solid tumors using tumor-infiltrating lymphocytes (TILs). This cutting-edge therapy involves surgically extracting T cells from a patient’s tumor, multiplying them in a laboratory, and then reintroducing them to the patient’s body to more effectively target and kill cancer cells. Even more exciting, this is a one-time treatment that can work for patients even when standard immunotherapy with checkpoint inhibitors has failed.
Stanford recently marked a significant milestone under Betof Warner’s leadership: becoming the first center to treat a patient with this innovative cell therapy following FDA approval. This achievement highlights the rapid progress of Betof Warner’s team and the potential of TIL therapy to provide a powerful new treatment option for cancer patients.
Stanford has been a leader in cell therapy for hematological malignancies for many years. “The opportunity to bring that expertise to a whole new population of patients is what brought me here,” Betof Warner states. “It was crucial to me that if I came here to build this program, we could start treating patients with urgency as soon as FDA approval was granted.”
Betof Warner and her team scheduled the first surgery within hours of the FDA approval. “We had a wait list of patients,” she recalls. “Being able to immediately make those phone calls after FDA approval and say, ‘I know you’ve been waiting, and here’s the day of your surgery,’ was a huge milestone.”
Trying a new therapy is both exciting and daunting. “We want to ensure that we’re doing the right thing for the patients, but we knew we had the pieces in place here at Stanford to do it,” Betof Warner says. Now, she and her team have a full pipeline of patients.
The Future of Outpatient Cancer Treatment: Toward Safer, More Effective Approaches
Despite the innovative approach, the success rate with TIL therapy currently stands at only 30% to 50%. A limited number of patients respond to TIL therapy, and many patients are still ineligible for this treatment due to various health conditions or the extent of their disease.
“We need to do better,” Betof Warner asserts. Together with her team, she is working to develop enhanced TILs that can benefit more patients and deliver safer treatment outcomes.
Betof Warner’s lab continuously collects blood, tumor biopsies, and cell samples from every patient treated who is willing. They are investigating what makes the best TILs by identifying characteristics that may predict a patient’s response to therapy. One major development is engineering TILs to function without interleukin-2 (IL-2), a cytokine essential for T-cell activation but notorious for severe side effects, limiting treatment eligibility.
Betof Warner’s team recently launched a clinical trial for an IL-2–independent TIL therapy. This new approach expands treatment to patients with other cancers and promises to make the therapy more accessible and less taxing. In developing TILs that do not require hospitalization for IL-2 administration, the goal is to transform TIL therapy into a fully outpatient treatment, allowing patients to maintain normalcy while undergoing therapy.
“We’re pushing the envelope,” Betof Warner says. “Our goal is to make TIL therapy safer, more inclusive, and ultimately more effective – offering patients significantly more hope.”