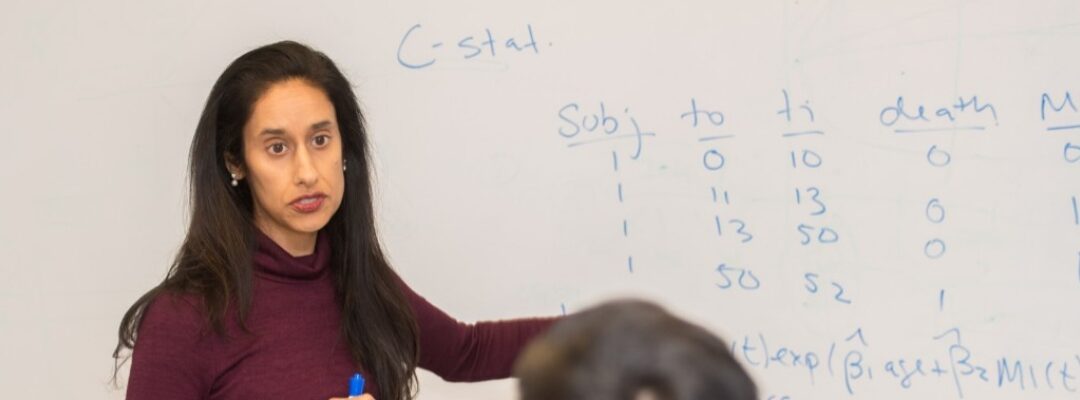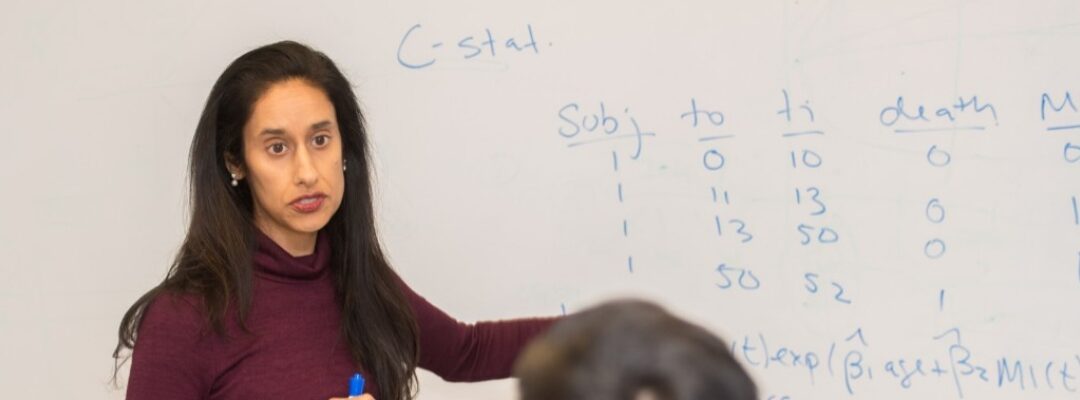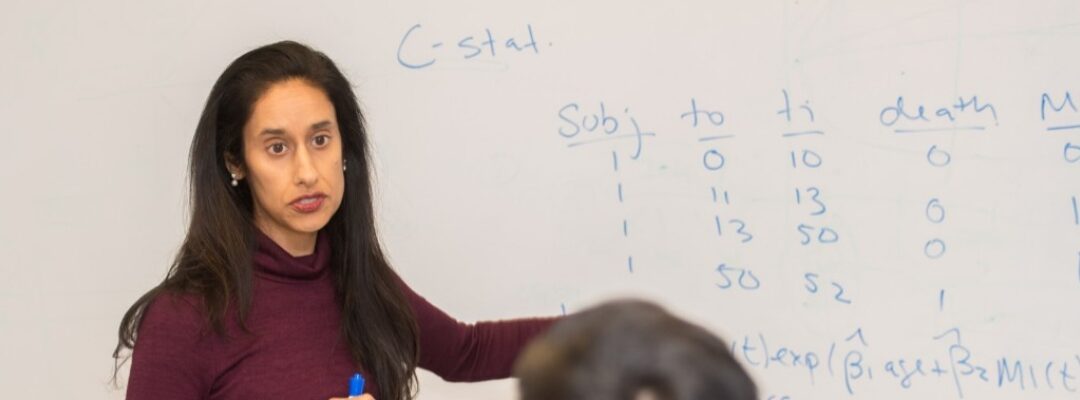
by emli1120 | Feb 26, 2024 | 2018, creating 2018
Manisha Desai, PHD, Professor of Biomedical Informatics Research Quantitative Sciences Unit: It’s Not About the Sample Size Manisha Desai, PHD, Professor of Biomedical Informatics Research Quantitative Sciences Unit: It’s Not About the Sample Size...

by emli1120 | Feb 26, 2024 | 2018, creating 2018
Ami Bhatt, MD, PhD The New Stanford Center for Arrhythmia Research: A Multidisciplinary Approach at Heart Ami Bhatt, MD, PhD The New Stanford Center for Arrhythmia Research: A Multidisciplinary Approach at Heart The Division of Cardiovascular Medicine has launched the...

by emli1120 | Feb 26, 2024 | 2018, creating 2018
Pamela Kunz, MD, assistant professor of medicine and director of the Neuroendocrine Tumor Program Conversations on Combating Cancer Pamela Kunz, MD, assistant professor of medicine and director of the Neuroendocrine Tumor Program Conversations on Combating Cancer In...
by emli1120 | Feb 26, 2024 | 2018, creating 2018
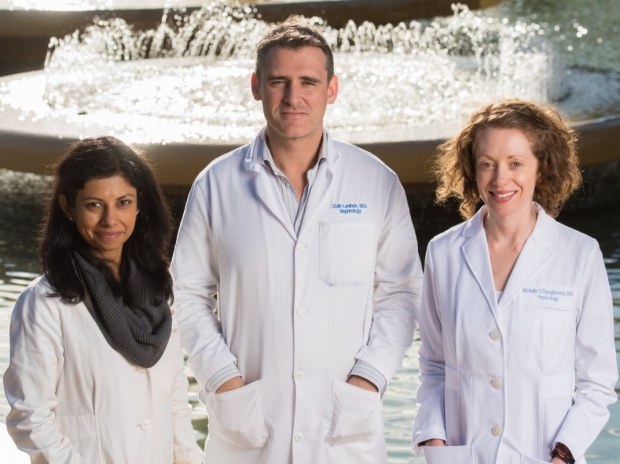
From left: Drs. Shuchi Anand, Colin Lenihan, and Michelle O’Shaughnessy are addressing some of nephrology’s toughest challenges. Young Nephrologists Asking Big Questions About Kidney Diseases From left: Drs. Shuchi Anand, Colin Lenihan, and Michelle...

by emli1120 | Feb 26, 2024 | 2018, sharing 2018
Lisa Shieh, MD, PhD (right), makes a point about quality improvement with medical residents Residents’ Elective Tackles Quality Improvement Research Lisa Shieh, MD, PhD (right), makes a point about quality improvement with medical residents Residents’ Elective Tackles...

by emli1120 | Feb 26, 2024 | 2018, sharing 2018
Chad Weldy, MD, PhD The Tipping Point: How Stanford’s Translational Investigator Program Supports—and Propels—the Careers of Early Physician-Scientists Chad Weldy, MD, PhD The Tipping Point: How Stanford’s Translational Investigator Program Supports—and Propels—the...